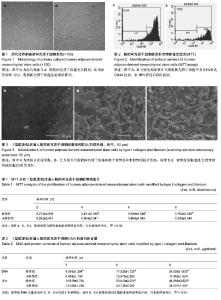
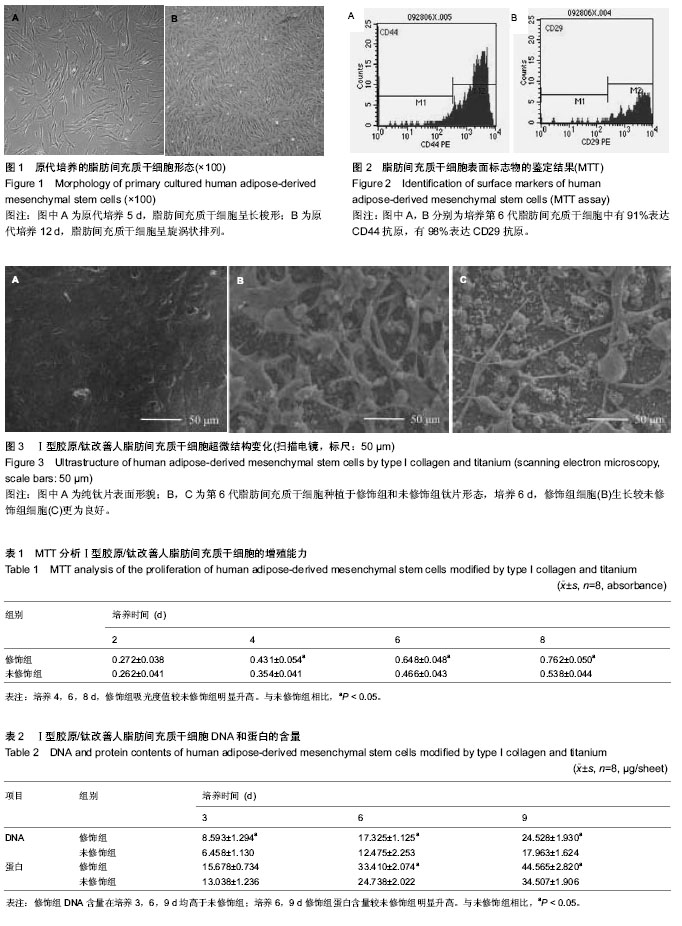
| [1] Frosch KH, Drengk A, Krause P, et al. Stem cell-coated titanium implants for the partial joint resurfacing of the knee. Biomaterials. 2006;27(12):2542-2549.
[2] 栗兴超,董福生.种植体表面优化处理对其骨结合促进的研究进展[J].现代口腔医学杂志,2007,21(6):645-646.
[3] 吴应龙,梁剑秋,刘族志.纯钛金属种植体表面活性化的处理方法[J].中国组织工程研究与临床康复,2008,12(14):2693-2696.
[4] 庄燕燕,胡仁,陈菲,等.钛植入体表面生物化学改性的研究进展[J].生物医学工程学杂志,2005,22(3):618-621.
[5] Morra M. Biochemical modification of titanium surfaces: peptide and ECM proteins. Eur Cell Mater. 2006;12:1-15.
[6] Roehlecke C, Witt M, Kasper M. Synergistic effect of titanium alloy and collagen type I on cell adhesion, proliferation and differentiation of osteoblast-like cells. Cells Tissues Organs. 2001;168(3):178-187.
[7] Schliephake H, Scharnweber D, Roesseler S, et al. Biomimetic calcium phosphate composite coating of dental implants.Int J Oral Maxillofac Implants. 2006;21(5):738-746.
[8] Sumner DR, Turner TM, Urban RM, et al. Additive enhancement of implant fixation following combined treatment with rhTGF-beta2 and rhBMP-2 in a canine model. J Bone Joint Surg Am. 2006;88(4):806-817.
[9] Lamberg A, Schmidmaier G, Soballe K. Locally delivered TGF-beta1 and IGF-1 enhance the fixation of titanium implants: a study in dogs. Acta Orthopaedica. 2006;77(5): 799-805.
[10] Schliephake H, Scharnweber D, Dard M, et al. Functionalization of dental implant surfaces using adhesion molecules. J Biomed Mater Res B Appl Biomater. 2005;73(1): 88-96.
[11] Huang ZM, Qi YY, Du SH, et al. Promotion of osteogenic differentiation of stem cells and increase of bone-bonding ability in vivo using urease-treated titanium coated with calcium phosphate and gelatin. Sci Technol Adv Mater. 2013; 14(5):055001.
[12] Sawada R, Kono K, Isama K, et al. Calcium-incorporated titanium surfaces influence the osteogenic differentiation of human mesenchymal stem cells. J Biomed Mater Res A. 2013;101(9):2573-2585.
[13] Guida L, Annunziata M, Rocci A, et al. Biological response of human bone marrow mesenchymal stem cells to fluoride-modified titanium surfaces. Clin Oral Implants Res. 2010;21(11):1234-1241.
[14] Schliephake H, Scharnweber D. Chemical and biological functionalization of titanium for dental implants. J Mater Chem. 2008;18:2404-2414.
[15] Yang J, Wang J, Yuan T, et al. The enhanced effect of surface microstructured porous titanium on adhesion and osteoblastic differentiation of mesenchymal stem cells. J Mater Sci Mater Med. 2013;24:2235-2246
[16] 黄怡,李源莹,杨成,等.骨髓间充质干细胞与不同表面处理钛片的生物相容性研究[J].华中科技大学学报(医学版),2010,39(5): 658-662.
[17] 吕仁发,周强.胶原在骨组织工程应用中的体外制备及生物相容性评价[J].中国临床康复,2005,9:194-195.
[18] Noah EM, Chen JS, Jiao XY, et al. Impact of sterilization on the porous design and cell behavior in collagen sponges prepared for tissue engineering. Biomaterials. 2002;23: 2855-2861.
[19] Berglund JD, Mohseni MM, Nerem RM, et al. A biological hybrid model for collagen 2 based tissue engineered vascular constructs. Biomaterials. 2003;24:1241-1254.
[20] 王玉丽.胶原在生物医学中的应用[J].国外医学:药学分册, 2002, 29:113.
[21] 张云松,高建华,鲁峰,等.Ⅰ型胶原支架材料与人脂肪干细胞体外生物相容性研究[J].南方医科大学学报,2007,27(2):223-225.
[22] 李力群,高建华,鲁峰,等.人脂肪来源干细胞与Ⅰ型胶原支架体外复合培养的研究[J].中华实验外科杂志,2012,29(3):421-423.
[23] 范利梅,唐旭炎,李全利,等.骨髓基质细胞在胞外基质涂层钛表面的黏附和铺展[J].中国组织工程研究与临床康复, 2009,13(21): 4029-4032.
[24] 贾帅军,孟国林,刘建,等.胶原修饰快速成形PLGA/TCP人工骨支架体外生物相容性研究[J]科学技术与工程,2009,12:336-339.
[25] Bagno A, Piovan A, Dettin M, et al. Human osteoblast-like cell adhesion on titanium substrates covalently functionalized with synthetic peptides. Bone. 2007;40(3):693-699.
[26] Ramims PA, Ciuffrida A, Milella E, et al. Three dimensional reconstruction of confocall laser microscopy images to study the behaviour of osteoblastic cells grown on biomaterials. Biomaterials. 2002;23(2):397-406.
[27] 陈竹生,吕玉明,张兵.纳米β-磷酸三钙/Ⅰ/Ⅱ型胶原层状支架复合骨髓间充质干细胞修复膝关节骨软骨缺损[J].中国组织工程研究,2010,14(42):7797-7801.
[28] 贺冬冬,曾令员,向川,等.Ⅰ/Ⅲ型胶原膜联合自体骨髓间充质干细胞修复兔膝关节软骨缺损[J].中国组织工程研究, 2012, 16(38): 7031-7036.
[29] Catherine DR, Timothy AP, Kellie LB. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials. 2007;28(21):3228-3235.
[30] Ye C, Hu P, Ma M, et al. PHB/PHBHHx scaffolds and human adipose-derived stem cells for cartilage tissue engineering. Biomaterials. 2009;30(7):4401-4406.
[31] Liu J, Zhao B, Zhang Y, et al. PHBV and predifferentiated human adipose-derived stem cells for cartilage tissue engineering. J Biomed Mater Res A. 2010;94(2):603-610. |